Carboxymethyl cellulose
Chemistry
Cellulose is a natural molecule, that consists of numerous residues of anhydroglucose, everyone carrying three reactive hydroxyl groups: by replacing one or more of cellulose's hydroxyls with carboxymethyl groups by etherification, water soluble CMC is obtained.
The chemical modification runs through two steps: the first step is the treatment of the cellulose with caustic soda to break the crystalline clusters and obtain the alkali-cellulose complex.
The second step is the etherification reaction between the alkali-cellulose complex and monochloroacetic acid promptly converted into sodium monochloroacetate with consequent formation of CMC, sodium chloride and sodium glycolate.
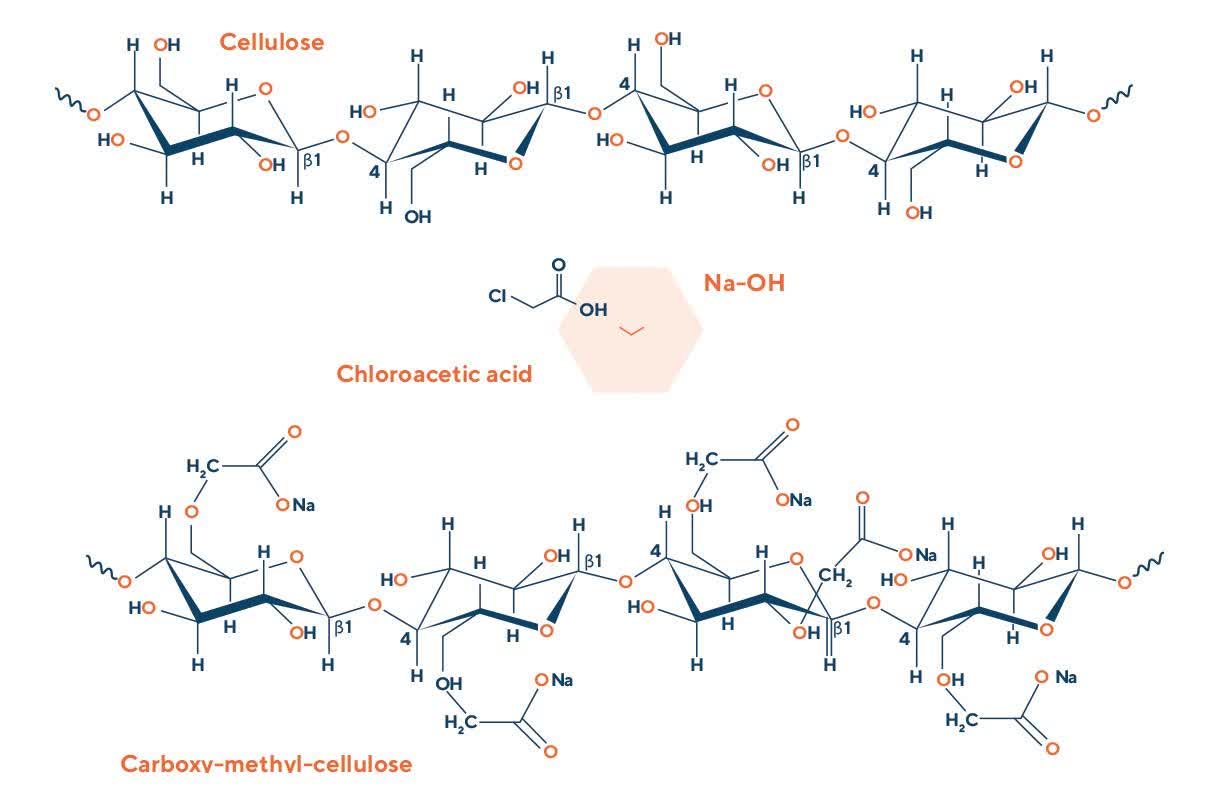
After the reaction sodium chloride, sodium glycolate and other impurities can be removed by treatment with an aqueous solvent to obtain pure grade CMC.
We manufacture a wide range of CMC grades, varying both the length of the polymer backbone and the number of substitutive carboxylic derivative, providing different levels of viscosity and tuning the several properties of the polymer.
